Phosphorus Tribromide
Phosphorus Tribromide Specification
- Density
- 2.85 Gram per cubic centimeter(g/cm3)
- Melting Point
- -41.5C
- HS Code
- 28112900
- Solubility
- Decomposes in water, soluble in organic solvents like carbon disulfide and benzene
- Molecular Weight
- 270.69 g/mol
- Smell
- Pungent, irritating odor
- Structural Formula
- Br3P
- Form
- Fuming Liquid
- Refractive Rate
- 1.597 (20C)
- Poisonous
- Yes
- Purity
- >99%
- Boiling point
- 173.2C
- Other Names
- PBr3
- Shape
- Liquid
- Storage
- Store in tightly closed containers in a cool, well-ventilated area, away from moisture
- Molecular Formula
- PBr3
- Classification
- Halide
- Chemical Name
- Phosphorus Tribromide
- CAS No
- 7789-60-8
- EINECS No
- 233-780-1
- Grade
- Industrial Grade
- Standard
- Complies with industrial chemical standards
- Type
- Inorganic Chemical
- Usage
- Pharmaceutical intermediates, agrochemicals, dye and pigment manufacture, chemical synthesis
- Main Material
- Phosphorus Tribromide (PBr3)
- Application
- Used as a reagent for introducing bromine into organic compounds, and in the preparation of organophosphorus compounds
Phosphorus Tribromide Trade Information
- FOB Port
- Mumbai
- Payment Terms
- Cash Advance (CA)
- Main Export Market(s)
- Asia
- Main Domestic Market
- All India
About Phosphorus Tribromide
Our Company holds immense experience in this domain, engaged in offering our customers a broad scope of Phosphorus Tribromide. Phosphorus tribromide is a colourless to pale yellow orange yellow fuming liquid with the formula Pbr3 and molecular weight is 270.7. It fumes in air due to hydrolysis and has a penetrating odour. It is widely used in the laboratory for the conversion of alcohols to alkyl bromides. Besides, we make this product checked by our strict quality controllers before delivering at the customers end.
Features:
- Melting Point(deg. C) -40
- Boiling Point(deg. C) 175/760 mm
- Safe to use
- Free from impurities
Key Features and Specifications
Phosphorus Tribromide is characterized by its colorless to yellow fuming liquid appearance, high purity (>99%), and strong pungent smell. The compound decomposes in water, forming phosphorous acid and hydrobromic acid, and is soluble in organic solvents like carbon disulfide and benzene. Its structural formula is Br3P, and it complies with industrial chemical standards for quality and safety.
Safe Handling and Storage
Due to its corrosive and poisonous properties, PBr3 must be handled with extreme care, utilizing protective clothing and equipment. The substance should be stored in tightly sealed steel or glass-lined containers, in cool, well-ventilated areas, away from moisture. Following these guidelines ensures stability for up to two years and minimizes risks of environmental hazards.
Applications and Industrial Uses
Phosphorus Tribromide serves an essential role in chemical synthesis, particularly for introducing bromine into organic compounds. Its versatility extends to pharmaceutical intermediates, agrochemicals, and the manufacturing of dyes and pigments. As an industrial-grade halide, its reliability and reactivity make it crucial across multiple chemical sectors.
FAQs of Phosphorus Tribromide:
Q: How should Phosphorus Tribromide be safely handled during use?
A: Phosphorus Tribromide demands careful handling due to its corrosive and poisonous nature. Always wear protective clothing, gloves, and eye protection, and work in a well-ventilated area. Avoid exposure to moisture and water as it reacts violently.Q: What is the recommended storage process for Phosphorus Tribromide?
A: Store Phosphorus Tribromide in tightly sealed bottles or drums made from steel or glass-lined materials. Containers must be kept in a cool, dry, and well-ventilated space, away from moisture to prevent decomposition and ensure a safe environment.Q: When does Phosphorus Tribromide decompose, and what are the by-products?
A: Phosphorus Tribromide decomposes upon contact with water, producing phosphorous acid and hydrobromic acid. This decomposition is rapid and exothermic, making it critical to prevent exposure to moisture during handling and storage.Q: Where can Phosphorus Tribromide be applied in industry?
A: PBr3 is widely used in pharmaceutical intermediates, agrochemical synthesis, dye and pigment production, and general organic synthesis, particularly for introducing bromine into compounds. Its role is integral within chemical manufacturing processes.Q: What benefits does Phosphorus Tribromide offer in chemical synthesis?
A: Phosphorus Tribromide is highly valued for its efficiency in introducing bromine atoms into organic molecules, facilitating the production of organophosphorus compounds and enhancing the versatility of chemical synthesis in industrial applications.Q: How does Phosphorus Tribromide impact the environment, and what precautions are necessary?
A: Phosphorus Tribromide is hazardous to aquatic life. To mitigate environmental risks, avoid releasing the compound into water sources, and adhere strictly to safe handling, storage, and disposal procedures as outlined by chemical safety regulations.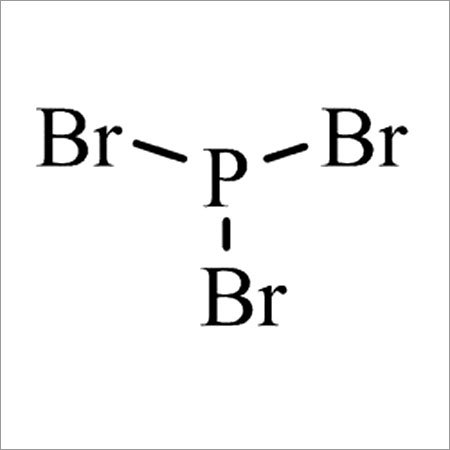
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email


 Send Inquiry
Send Inquiry

