Methyl Bromoacetate
Methyl Bromoacetate Specification
- Poisonous
- Yes
- Refractive Rate
- 1.447 to 1.449
- Density
- 1.595 Gram per cubic centimeter(g/cm3)
- Smell
- Pungent, ester-like odor
- Molecular Weight
- 152.98 g/mol
- Purity
- 99%
- Storage
- Store in a cool, dry, well-ventilated area away from incompatible substances
- Form
- Clear liquid
- Structural Formula
- BrCH2COOCH3
- Shape
- Liquid
- Melting Point
- -51 C
- Molecular Formula
- C3H5BrO2
- Boiling point
- 144-145 C
- Solubility
- Soluble in water, alcohol, and ether
- Other Names
- Bromoacetic acid methyl ester
- Taste
- Not determined; toxic
- HS Code
- 29153990
- Classification
- Alkylating agent, Ester
- Chemical Name
- Methyl Bromoacetate
- CAS No
- 96-32-2
- EINECS No
- 203-483-6
- Grade
- Industrial Grade
- Standard
- Meets industry standards
- Type
- Organic chemical compound
- Usage
- Laboratory chemicals, synthesis of pharmaceuticals and agrochemicals
- Main Material
- Methyl Bromoacetate
- Application
- Used as an alkylating agent, in pharmaceuticals and organic synthesis
- Flash Point
- 58 °C (closed cup)
- Appearance
- Colourless to pale yellow liquid
- Packaging
- Sealed containers, bottles, or drums
- Vapor Pressure
- 1.3 mmHg at 20 °C
- Shelf Life
- 2 years under proper storage
- Autoignition Temperature
- 415 °C
- Stability
- Stable under recommended storage conditions
- Precautions
- Use with adequate ventilation; avoid inhalation and contact
Methyl Bromoacetate Trade Information
- FOB Port
- Mumbai
- Payment Terms
- Cash Advance (CA)
- Main Export Market(s)
- Asia
- Main Domestic Market
- All India
About Methyl Bromoacetate
We are leading manufacturers, exporters and suppliers of a comprehensive range of quality Methyl Bromoacetate for our valued patrons from Maharashtra, India. Highly corrosive in nature, these chemicals find extensive usage in different industries. This is a colorless to light yellow liquid and us used as the building block intermediate in various pharmaceutical molecules. Methyl Bromoacetate has molecular formula BrCH2CO2CH3, Molecular weight 152.98 and boiling point(deg .C)-51 to 52/15 mm.
Features:
- M.W.: 152.98
- BP: 51 - 52 C(15mm)
- Density: 1.616
- Purity: >= 98%(GC)
Highly Versatile Alkylating Agent
Methyl Bromoacetate is trusted in the synthesis of pharmaceuticals, laboratory chemicals, and agrochemicals due to its high purity and reactivity. Its solubility in water, alcohol, and ether makes it suitable for a wide array of organic transformations, providing chemists with flexibility in various processes.
Safe Storage and Handling
Maintaining product integrity relies on storing Methyl Bromoacetate in sealed containers away from incompatible substances. The liquid remains stable for up to two years in well-ventilated, cool, and dry areas. To ensure safety, always use appropriate protective equipment and avoid direct inhalation or skin contact.
Application Excellence Across Industries
This industrial-grade methyl bromoacetate meets industry standards and is supplied by exporters and manufacturers in India. Its key benefits include consistent quality for reliable synthesis results in research institutes and industrial laboratories worldwide.
FAQs of Methyl Bromoacetate:
Q: How should Methyl Bromoacetate be safely stored to maintain its stability?
A: Store Methyl Bromoacetate in sealed bottles or drums in a cool, dry, and well-ventilated area away from incompatible materials. Proper storage under recommended conditions ensures the compound remains stable and effective for up to two years.Q: What are the primary uses of Methyl Bromoacetate in industry?
A: Methyl Bromoacetate is predominantly used as an alkylating agent in laboratory research, the synthesis of pharmaceuticals, and the preparation of agrochemicals. Its chemical properties enable efficient incorporation into complex synthetic processes.Q: When should extra precautions be taken during the handling of Methyl Bromoacetate?
A: Extra precautions are necessary whenever handling, transferring, or disposing of the compound. Use personal protective equipment, ensure adequate ventilation, and avoid inhalation or direct skin contact due to its toxic nature and pungent odor.Q: Where can Methyl Bromoacetate be applied for maximum utility?
A: Methyl Bromoacetate is most beneficial in organic chemical synthesis, particularly for producing pharmaceutical intermediates and agrochemical compounds in laboratory and industrial settings.Q: What is the process for using Methyl Bromoacetate in organic synthesis?
A: This compound is commonly introduced as a methylating or alkylating agent in various organic reactions. It reacts with nucleophiles, such as amines or alcohols, to form corresponding esters or ethers under controlled laboratory conditions.Q: How does Methyl Bromoacetate benefit pharmaceutical and chemical synthesis?
A: Its high purity and predictable reactivity contribute to efficient, high-yield syntheses, enhancing reliability and reproducibility in the production of valuable intermediates for pharmaceuticals and agrochemicals.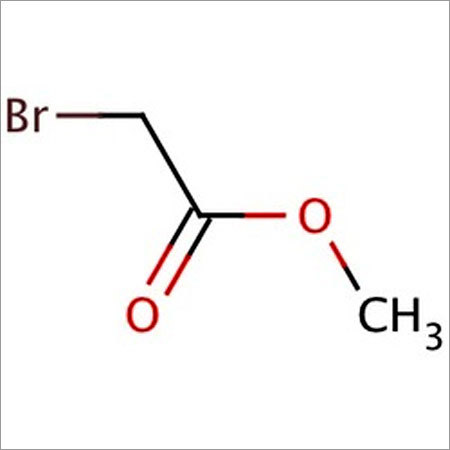
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email


 Send Inquiry
Send Inquiry

