Bromoform
Bromoform Specification
- Structural Formula
- H-C(Br)(Br)-Br
- Ph Level
- Neutral
- Density
- 2.89 Gram per cubic centimeter(g/cm3)
- Storage
- Store in a cool dry and well-ventilated area away from flames and heat.
- Other Names
- Tribromomethane
- Purity
- 99%
- Refractive Rate
- 1.595
- Melting Point
- 8.3C
- Solubility
- Slightly soluble in water; soluble in ethanol ether and chloroform.
- Molecular Weight
- 252.73 g/mol
- Form
- Clear to yellowish liquid
- Poisonous
- No
- HS Code
- 290339
- Shape
- Liquid
- Molecular Formula
- CHBr3
- Boiling point
- 149.5C
- Smell
- Sweet chloroform-like odor
- Classification
- Halogenated Hydrocarbons
- Chemical Name
- Bromoform
- CAS No
- 75-25-2
- EINECS No
- 200-856-7
- Grade
- Industrial Grade
- Standard
- ISO 9001
- Type
- Chemical Compound
- Usage
- Used as a laboratory reagent flame retardant and solvent; also employed in the synthesis of other chemicals.
- Main Material
- Bromine
- Application
- Industrial cleaning agents flame retardants and chemical research.
About Bromoform
Our domain expertise has enabled us to come up with a comprehensive range of excellent quality Bromoform. In the past, it was used as a solvent, flame retardant and sedative, but now it is mainly used as a laboratory reagent. It is a pale yellowish liquid with a sweet odor similar to chloroform, a halomethane or haloform with molecular formula CHBr3. This bromoform is highly acknowledged among the customers for their quality characteristics such as precise non-toxicity, pH value and environment friendliness.
Features:
- Assay 99+%
- Density 2.894
- Accurate composition
- Longer shelf life
Chemical Properties and Structure
Bromoform, also known as tribromomethane, possesses the structural formula H-C(Br)(Br)-Br and presents a boiling point of 149.5C with a melting point at 8.3C. The compound has a refractive index of 1.595 and is recognized for its high purity (99%) in industrial applications. Its distinct sweet odor and clear to yellowish appearance identify it for both laboratory and manufacturing processes.
Applications and Industrial Usage
Primarily utilized as a laboratory reagent, Bromoform is essential in various chemical syntheses and research activities. Its flame retardant properties make it valuable for industrial protection, while its solvency enhances effectiveness in cleaning agents and other chemical formulations. The compounds versatility ensures its prevalence in multiple sectors from research institutions to manufacturing facilities.
Storage and Safety Guidelines
Due to its chemical nature, Bromoform must be stored in cool, dry, and well-ventilated environments, away from any sources of heat or open flames, ensuring optimal safety. Proper storage maintains product integrity and mitigates potential risks, especially given its classification as a halogenated hydrocarbon. Compliance with ISO 9001 standards is recommended for handling and storage.
FAQs of Bromoform:
Q: How should Bromoform be safely stored in an industrial setting?
A: Bromoform should be stored in a cool, dry, and well-ventilated area, kept away from direct sunlight, flames, and sources of heat to prevent any hazardous reactions. Adherence to storage guidelines ensures product stability and safety.Q: What are the primary uses of Bromoform in industry?
A: Bromoform is mainly used as a laboratory reagent, flame retardant, and solvent. Additionally, it plays a critical role in the synthesis of various chemicals and is sometimes incorporated into industrial cleaning agents.Q: When is Bromoform typically utilized in chemical research?
A: Bromoform is employed in chemical research when a dense, halogenated solvent or reagent is needed, particularly in organic synthesis, extraction processes, or when evaluating flame-retardant compounds.Q: Where does Bromoform find application outside laboratory environments?
A: Beyond laboratories, Bromoform is used as a flame retardant in manufacturing settings and as a solvent in the industrial production of other chemicals, notably in cleaning agent formulations and specialized industrial processes.Q: What is the process for handling accidental spills of Bromoform?
A: In the event of a spill, ventilate the affected area, contain and collect the liquid using appropriate absorbent materials, and dispose of it according to chemical safety regulations. Use personal protective equipment during cleanup to avoid exposure.Q: How does Bromoform benefit industrial processes?
A: Its high density and solvency enhance reaction efficiency, while its flame-retardant properties contribute to workplace safety. These characteristics make Bromoform a reliable choice for various industrial and research functions.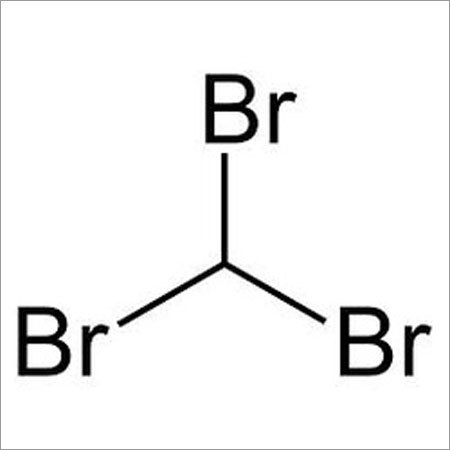
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email


 Send Inquiry
Send Inquiry

