2 Bromopropionic Acid
2 Bromopropionic Acid Specification
- Structural Formula
- BrCH2CHCOOH
- HS Code
- 291830
- Refractive Rate
- 1.512
- Smell
- Characteristic pungent odor
- Boiling point
- 145C (at 20 mmHg)
- Molecular Weight
- 152.98 g/mol
- Molecular Formula
- C3H5BrO2
- Classification
- Organic acid
- Organic Acid Types
- Carboxylic Acid
- Grade
- Industrial Grade
- Purity
- 99%
- Application
- Intermediate in organic synthesis; starting material in the production of pharmaceuticals and agrochemicals
- Appearance
- Colorless to pale yellow liquid or solid
- CAS No
- 598-72-1
- EINECS No
- 209-962-5
- Other Names
- -Bromopropionic acid
- Usage
- Used as a reagent for various chemical reactions and synthesis in labs and industries
- Melting Point
- 37C
- Density
- 1.77 Gram per cubic centimeter(g/cm3)
- Solubility
- Soluble in water and organic solvents
- Raw Material
- Starting materials for brominated intermediates
About 2 Bromopropionic Acid
Backed by the state-of-art infrastructure, we offer the fast acting chemical 2- Bromo Propionic Acid from Maharashtra, India. It is clear colorless to yellowish liquid after melting with molecular formula CH3CHBrCOOH. Our product is widely used in various scientific researches and synthesis of pharmaceuticals and pesticides. Our offered products 2Bromopropionic acid is supplied to customers at reasonable prices within the stipulated time frame.
Features:
- Clear to yellow liquid
- Water Solubility : soluble
- Boiling point - 200 - 205 C
- Specific Gravity 1.700W/V
Versatile Intermediate for Synthesis
2-Bromopropionic Acid is renowned for its efficacy as an intermediate in organic synthesis. Its unique structure and reactivity make it a valuable starting material for the preparation of brominated compounds used in pharmaceuticals and agrochemicals. The products high purity ensures reliability and consistent results across diverse chemical reactions.
Key Properties and Specifications
This carboxylic acid features a molecular weight of 152.98 g/mol, a refractive rate of 1.512, and a prominent melting point at 37C. Recognized for its solubility in both water and organic solvents, 2-Bromopropionic Acid offers flexibility in a variety of laboratory and industrial chemical processes. Its distinct pungent odor and physical properties are characteristic of high-grade organic acids.
Applications Across Industries
Used predominantly as a reagent and intermediate, 2-Bromopropionic Acid is indispensable in chemical manufacturing. It serves as a precursor in the synthesis of pharmaceuticals, specialty chemicals, and crop protection agents. Exported, manufactured, and supplied in India, this organic acid fulfills the stringent quality expectations of both local and global markets.
FAQs of 2 Bromopropionic Acid:
Q: How is 2-Bromopropionic Acid typically used in industrial applications?
A: 2-Bromopropionic Acid is primarily utilized as an intermediate in the synthesis of pharmaceuticals and agrochemicals. Its high reactivity and purity make it ideal for producing brominated compounds and other complex organic molecules in industrial processes.Q: What benefits does 2-Bromopropionic Acid offer as a reagent or starting material?
A: The main advantages are its high purity (99%), excellent solubility in water and organic solvents, and consistent quality. These features ensure reliable chemical reactions, high product yields, and versatile use across different industrial and laboratory settings.Q: When should 2-Bromopropionic Acid be chosen over other brominated acids?
A: It is most suitable when a carboxylic acid group with a bromine substituent at the alpha position is needed, particularly in syntheses where this configuration enhances reactivity or selectivity in pharmaceutical or agrochemical routes.Q: Where is 2-Bromopropionic Acid manufactured and supplied from?
A: This product is manufactured, supplied, and exported from India by specialized chemical producers, catering to both domestic and international markets looking for high-grade organic intermediates.Q: What safety considerations should be observed when handling 2-Bromopropionic Acid?
A: Use appropriate protective equipment such as gloves, goggles, and lab coats. The substance has a pungent odor and may emit fumes, so employ proper ventilation. Follow standard chemical handling and storage protocols to ensure safety.Q: How is 2-Bromopropionic Acid processed or purified after synthesis?
A: Following its chemical synthesis, 2-Bromopropionic Acid undergoes purification steps such as distillation under reduced pressure and recrystallization, ensuring a purity level of at least 99% suitable for industrial and laboratory applications.Q: What are the primary benefits of using 2-Bromopropionic Acid in the synthesis of pharmaceuticals and agrochemicals?
A: Its reliable structure as a brominated carboxylic acid enhances molecular complexity, facilitating the creation of advanced active ingredients for pharmaceuticals and crop protection, while its high purity aids in achieving precise reaction outcomes.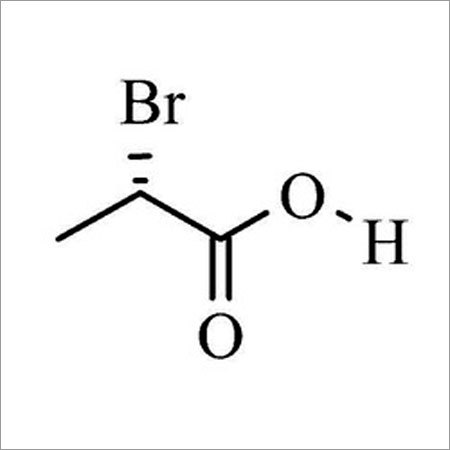
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email


 Send Inquiry
Send Inquiry

